Electrochemical access to benzimidazolone and quinazolinone derivatives via in situ generation of isocyanates†
Abstract
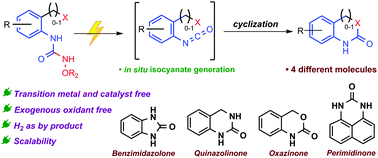
Isocyanates are the key intermediates for several organic transformations towards the synthesis of diverse pharmaceutical targets. Herein, we report the development of an oxidant-free protocol for electrochemical in situ generation of isocyanates. This strategy highlights expedient access to benzimidazolones and quinazolinones and eliminates the need for exogenous oxidants. Furthermore, detailed mechanistic studies provide strong support towards our hypothesis of in situ isocyanate generation.


 Please wait while we load your content...
Please wait while we load your content...